The Cloning of Dolly
Dr Jamie Love 27 November 1997 -2009 ©
27 November 1997 -2009 ©
 27 November 1997 -2009 ©
27 November 1997 -2009 ©
I've decided to make cloning one of the first topics in this first
issue of Science Explained because the folks
who created Dolly are acquaintances of mine. (Yes, I am a name
dropper, aren't I?) A few kilometers from my home lives Dolly,
the world's first mammalian clone; not counting identical twins.
(They're clones too.)
What makes Dolly different from identical twins
is that she was grown from a cell taken from an ADULT animal!
Many bright, well-respected scientists said it couldn't be done.
Dr Ian Wilmut, who is in charge of the lab that created
Dolly, admits that he had his doubts. However the hard work and
imaginative thinking of his staff made it all possible.
|
How did they do it and what did they do?
First some background to teach you the basics of developmental
biology.
A cell from a frog's gut should always remain a frog-gut-cell because it has "differentiated". Differentiation is the natural process whereby cells specialize into a certain kind of cell. As a frog embryo grows and develops its cells differentiate into nerve cells, blood cells, fat cells and many other different kinds of cells. That's what differentiation is all about. Differentiation is important because without it an animal could never be anything but a blob of unspecialized cells. As a mass of embryo cells divide and differentiate they "create" the animal. This incredible process of differentiation turns zygotes into animals and it's all controlled by the genes. Although the exact process is still poorly understood, scientists agree that differentiation must have something to do with changes in the nucleus of cells. You may recall that the nucleus is the part of the cell containing the genetic material (the DNA all coiled up in organized structures called chromosomes). |
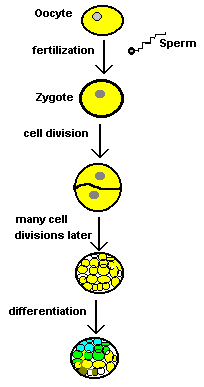
|
What do frog cells have to do with Dolly?
|
Like most scientific "breakthroughs" the earlier work
done by others provided the foundation on which to try something
new.
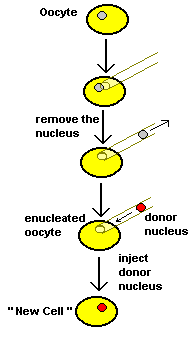
Decades ago a fellow named Gurdon developed the method of "nuclear transfer". This is a two step process. First he used delicate needles and a good microscope to suck out the nucleus from a frog oocyte, producing an "enucleated oocyte". (That's an oocyte without a nucleus.) With the genetic material removed the enucleated oocyte would not divide or differentiate even when fertilized. That was no surprise. (A cell is nothing without its nucleus.) But the results from Gurdon's second step shocked a lot of people! He used the same equipment and skill to transfer the nucleus from a frog's gut cell into an enucleated oocyte. That's nuclear transfer, the transfer of a nucleus from one cell to another, creating a "new cell" with a different nucleus. Many of these new cells which Gurdon created behaved like a zygote. They divided and divided and divided just like a normal developing embryo, producing a ball of cells. And this ball of cells differentiated! Nerve cells, skin cells, blood cells appeared just as they would in a normal embryo. After the normal length of time Gurdon had tadpoles! Because the tadpoles had all come from the gut cells of the same adult, they all had the same genetic material. So they were all clones, identical twins of each other. But unlike normal identical twins they were made from differentiated cells. |
Naturally this got a lot of scientists thinking about cloning. But there were two problems.
First, Gurdon's nuclear transferred tadpoles never grew into frogs! Other folks repeated his experiments and got similar results. Nuclear transfer couldn't clone frogs from frog cells; all you got were tadpoles. No one knew why. Even today, no one knows why the tadpoles made by nuclear transfer die instead of growing into frogs. Weird.
The second problem was that Gurdon's method seemed to work only with frogs (or perhaps I should say "tadpoles"). When scientists tried nuclear transfer with mice, cattle or indeed any mammal, they got nowhere. The "new cells" sometimes divided a few times, but not for long and none of them differentiated properly. You just couldn't clone mammals. By the early 1980's most scientists accepted the idea that something very special allows frogs to be (partially) cloned (into tadpoles). Whatever that process was, it was not found in mammals. The textbooks made it very clear. Differentiation was (sort of) reversible in frogs but not in mammals.
Bummer.
So what exactly did the scientists at the Roslin Institute do?
Well, Keith Campbell, a fellow working for Dr Wilmut, thought that maybe the cell cycle had something to do with this cloning trouble.
|
The cell cycle is often described as a circle of
cell life and division, but I think that can be a bit confusing
for some people, so let's try to remember that by "cycle"
we mean it happens again and again.
A cell divides into two "daughter cells" and both of these cells live, "eat", grow, copy their genetic material and divide again producing two more daughter cells. Because each daughter cell has a copy of the same genes in its nucleus, daughter cells are "clones" of each other just like identical twins. This "twining" goes on and on with each cell cycle. This is a natural process. The cell cycle fascinates biologists. Very fast cell cycles occur during development causing a single cell to make many copies of itself as it grows and differentiates into an embryo. Some very fast cell cycles also occur in adult animals. Hair, skin and gut cells have very fast cell cycles to replace cells that naturally die. And cancer is a disease caused by cells cycling out of control. It's no wonder that biologists think the cell cycle is so important. But there is a kind of "parking spot" in the cell cycle called "quiescence" (pronounced "kwee-S-ence"). A quiescent ("kwee-S-cent") cell has left the cell cycle, it has stopped dividing. Quiescent cells might reenter the cell cycle at some later time, or they might not. It depends on the type of cell. Most nerve cells stay quiescent forever. On the other hand, some quiescent cells may later reenter the cell cycle in order to make more cells. (For example, when a young girl starts to develop breasts.) |
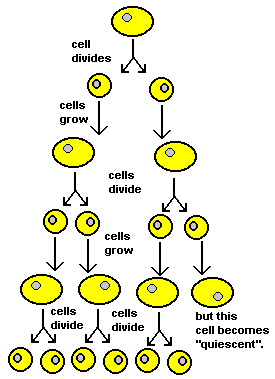
|
Many biologists (including myself) thought that to make a clone you should transfer the nucleus from a fast dividing cell. It made sense because fast cycling cells are exactly what makes an embryo grow. Besides, the gut cells used to make the tadpole clones were fast cycling cells. Many biologists tried to make clones by transferring the nucleus from fast dividing cells but all of those experiments were unsuccessful. (I tried injecting the fast growing cells from chicken feathers into hen eggs in the hope of cloning birds, but it didn't work.)
Keith (Dr Campbell) thought about it in a different way. He wondered
if a quiescent nucleus would be a better donor. True, it was not
cycling (that's what makes it quiescent, by definition) but Keith
thought maybe that's what the nucleus needs for it to be successfully
transferred. Maybe the cell needs time to "rest" before
starting to make a whole new animal. Maybe the nucleus needs time,
lots of time, to get its DNA in order. Maybe...?
Maybe quiescent cells would work!
So they tried it with cells from sheep.
The folks at the Roslin
Institute do a lot of work with sheep as part of their partnership
with a company called Pharmaceutical Proteins Limited Therapeutics
(PPL Therapeutics). Earlier they had made transgenic sheep (sheep
with human genes transferred into them, but that's another story).
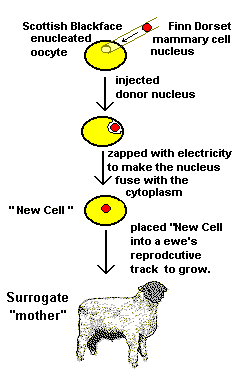
They used cells from an adult sheep's mammary (breast) glands
for the "donor" nucleus. They grew the cells in tissue
culture, an artificial situation that is commonly used in
laboratories to grow large numbers of cells in bottles. Tissue culture allows scientists to fiddle with
the cells and alter their characteristics. That is exactly what Dr
Campbell did. He "starved" the cells of important nutrients
and the cells stopped growing and dividing. They became quiescent.
(Keith knew they would become quiescent when starved of nutrients
because other researchers had proven that years ago; but few folks
really cared because who needs quiescent cells?)
|
And then he made Dolly?
Yes, but creating Dolly was not easy.
Oocytes have a "shell" of proteins and fibers (called the zona pellucida) and it is through this protective coat that Bill injected the nucleus from a quiescent mammary cell into the enucleated oocyte. That cell nucleus was from a different breed of sheep called a Finn Dorset, which happens to be a pure white breed of sheep. He then used a tiny pulse of electricity to cause the new nucleus to fuse with the enucleated oocyte's cytoplasm. (Cytoplasm is the solution inside the cell.) This electricity also helps "kick start" cells into "activity" so they are more likely to divide. This new, fused cell (containing the Finn Dorset mammary cell nucleus in the cytoplasm of a previously enucleated Blackface oocyte) was transferred into the reproductive "chamber" of a Blackface ewe (the same breed that provided the oocyte). |
Bill and his fellow researchers than repeated this process 276 times! That's right, 276 times.
I told you this wasn't easy.

|
After 148 days, a normal length of time for the Finn Dorset breed
of sheep, Dolly was born (5 July 1996).
As you can see she is a healthy, normal looking Finn Dorset. (Dolly's the wee one on the left) born to a Blackface ewe (her mom's on the right). This proves that Dolly wasn't the product of a sneaky mating; Dolly's Blackface mom could not produce a white faced sheep no matter who was the father. (It has to do with the genetics of sheep breeds.) But just to be sure, the scientists DNA "fingerprinted" Dolly and her "mom" and proved that Dolly's DNA matched the cells from the tissue culture, not the cells from the ewe that gave birth to her. Dolly is a normal (Finn Dorset) sheep. Contrary to the reports in some of the trash newspapers, she has not eaten her keeper or her fellow sheep. She does not shoot laser beams out of her eyes or talk. Dolly is a friendly, normal, healthy sheep who enjoys being petted, especially if you have some food in your hand! |


Return to the Science Explained Homepage.