 and
and  licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
licensed under a Creative Commons Attribution-ShareAlike 4.0 International License. and
and  licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.

|
|
 |
Atoms are the fundamental units of matter (and Chemistry) - the smallest unit of matter to keep its identity as a chemical element. Everything is made of a combination of atoms.
Elements are substances made of only one kind of atom (have the same number of protons, the same atomic number). Each element corresponds to a different kind of atom.
If you try to break up an element all you get are more of the same. You cannot divide an element into anything smaller (or it isn't an element, or even an atom, anymore).
Compounds are substances made of two or more kinds of atoms (with different numbers of protons, different atomic numbers).
74% of the universe is hydrogen (H), the first element. H has one proton.
24% of the universe is helium (He), the second element. He has two protons.
98% of the universe is hydrogen (H) and helium (He).
|
If you break up an atom you get: protons - a piece of matter with a positive charge (+1). electrons - an even smaller piece of matter with a negative charge (-1). neutrons - a piece of matter (about the same size as a proton) with no charge (0). Most atoms are circled by electrons. When the number of electrons equals the number of protons, the net charge is zero. Atoms in which the number of electrons is not equal to the number of protons are said to be ionized. These atoms are described as ions. If there are more electrons than protons, the ion has a negative charge. If there are more protons than electrons, the ion has a positive charge. The total charge is the sum of the positive protons and negative electrons. |

|
The number of protons defines the atom or element and gives it its chemical properties.
The atomic number is the number of protons in the atom.
The number of neutrons doesn't effect the atom's properties, except to give it extra mass.
Isotopes are elements with different numbers of neutrons, thus having different masses.
But isotopes of the same element always have the same number of protons, otherwise they'd be a different element.
Nucleons are the protons and neutrons combined.
Daltons are the fundamental unit of mass in Alchemy. Nucleons (protons and neutrons) all have a mass of one Dalton.
Electrons are so small that they are not even considered important in determining the atom's mass.
Atomic mass is the total mass of the atom. Because we ignore the mass of the electrons, the atomic mass is just the mass of the nucleons, each contributing one Dalton.
Relative atomic mass is a representation of the "average" mass of an element, taking into account all the element's isotopes and their relative abundance.
We abbreviate each element with one or two letters:
Hydrogen is H and Helium is He. Electrons are simply e.
We can state the atom's mass (nucleon count) immediately after the element's name. Like "helium-three" for a helium atom with three nucleons.
We write the number of nucleons to the upper left of the abbreviation like this: 3He.
We write the charge on an element or electron to the upper right of the abbreviation like this: e-
|
Hydrogen has three isotopes:
Normal Hydrogen is 1H, has just one proton (that's why it's hydrogen). Deuterium is 2H, has just one proton and a neutron. Tritium is 3H, has just one proton and two neutrons. Tritium is unstable. It is radioactive, spitting out a fast electron as it disintegrates (beta decays) into helium. 3H ------> 3He + e- |

|
A balanced equation has the same "net" charge and mass on the left side as on the right side.
Everything is the same on the two sides, just arranged differently.
There are three types of radiation:
Alpha particles are high speed helium nuclei (4He+2) which can be stopped by a piece of paper or skin.
Beta particles are high speed electrons (e-) which can pass thorough paper, but not a lot of paper such as a book.
Gamma rays are not particles at all. They are very high energy light rays that can only be stopped by very thick materials such as bricks.
Radiation can harm you if you get hit by too much of it!
|
Transmutation is when one element is turned into another (by having the number of its protons changed). Tritium is transmuted into helium when it beta decays.
Alpha decay also causes a transmutation. |

|
Half-life is the length of time required for half the amount of a radioisotope to decay. Each radioisotope has its own specific half-life. Nothing can change the rate at which a particular radioisotope decays. Half-lives are constant for each radioisotope.
We measure radioactivity in Curies (Ci), 1 Curie equals 37 billion disintegrations per second - the radioactivity of 1 gram of radium-226 (226Ra).
NOTE: I'm sad to report that in the 1990’s the scientific community began to switch over to another unit of radioactivity called the "Becquerel" (named after the fellow who discovered radioactivity). The good news is that the Becquerel (Bq) is easy to remember because 1 Bq = 1 disintegration per second (So 1 Curie is 37 billion Bq’s). Both Curies (Ci) and Becquerels (Bq) are used by chemists in the year 1999.
Specific activity is the number of Curies per gram of a substance (Ci/gram). One gram of radium-226 (226Ra) has a specific activity of 1 (Curie per gram).
NOTE: Because some people use Ci and others Bq it is important to explain which units YOU use when talking about specific activity. (Ci/gram or Bq/gram). Throughout this course (Principles of Alchemy) we will use Curies (because anyone can do the math for Becquerels!)
The universe started with the Big Bang. From this huge explosion came all the protons, neutrons and electrons.
The smallest elements, the first five, were made from the Big Bang and some are still being made inside of stars.
Stars squeeze atoms together, causing their nuclei to fuse in a process called nuclear fusion.
From normal nuclear fusion, we get all the elements up to iron (26 protons).
All the other elements (with more than 26 protons) were formed when these burned out stars exploded as nova.
Every atom in your body heavier than boron (atomic mass of 5), was once inside a star! (And most of the lighter ones were too.)
|
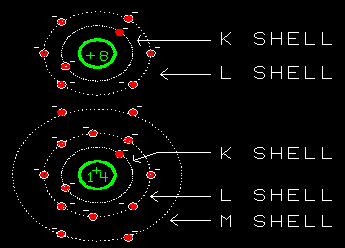
The shells (K, L, M and so on) define the distance of the electron cloud from the nucleus. They are fitted inside each other as a series of shells. The outer shell determines the size of an atom. Each larger shell can hold more electrons.
The K-shell can only hold 2 electrons.
All shells can be divided into subshells or orbitals that define the shape of the electron cloud. |
|
An electron cloud is the volume around the atom in which
its electrons are likely to be found. An atom's size (shell) and shape (orbitals)
are determined by its outer most electron cloud.
|
S orbitals have sphere-shaped electron clouds. Their electrons
are likely to be found in any direction from the nucleus
(that's a characteristic of an s orbital) at (roughly) a certain distance
from the nucleus (that's a characteristic of the shell).
All shells start with an s orbital, which is (also) always the lowest energy orbital of any shell. |

|
|
P orbitals have figure-8-shaped electron clouds. Their electrons
are restricted to specific directions causing them to have specific
shapes (figure-8) in specific directions. There are three types,
one for each dimension (x, y, z). Oxygen, carbon and nitrogen
are examples of atoms with p orbitals as their outer electron
cloud.
There are 5 types of d orbitals and 7 types of f orbitals.
Pauli's exclusion principle says no more than two
electrons may occupy a single orbital (subshell) and if two electrons do occupy a single orbital, their spins will be opposite to each other.
|

|
When assigning electrons, first fill all shells, starting
with the lowest energy shell (K) and working outward.
Then reassign electrons to the orbitals (subshells). Remember, only two electrons can fit in each orbital.
S orbitals are of lower energy than p orbitals so they are filled first. S orbitals are of only one type (you can only have one type of sphere) and can only be assigned two electrons (which will have opposite spins because of Pauli's exclusion principle).
Once the s orbital has been assigned two electrons, place any remaining electrons into p orbitals.
There are three types of p orbitals (x-p, y-p and z-p). Place one electron into each type of p orbital. (They will all have the same spin because of Hund's rule, either all "up" or all "down") Any remaining electrons can then be placed into these same p orbitals, forming electron pairs (which will have opposite spins, one will be "up" and the other will be "down").
All three of the p orbitals are of the same energy, so it doesn't matter which one gets pairs of electrons or remains empty.
Here's the electron configuration of some atoms to show how these laws work.
Hydrogen. K-shell (s1)
Helium. K-shell (s2)
Carbon. K-shell (s2), L-shell (s2, x-p1, y-p1)
(In this example the z-p orbital didn't get an electron, but it just as well could have been the x-p or y-p that remained empty.)
Also, note that the x-p and y-p electrons will either both be "up" or both be "down".
|
Oxygen. K-shell (s2), L-shell (s2, x-p2, y-p1, z-p1) (In this example the x-p orbital got two electrons, but it just as well could have been the x-p or y-p that got an electron pair.) Also, note that the two electrons in the x-p orbital will be of opposite spin, one will be "up" and the other will be "down". The electrons in y-p and z-p orbitals will both be parallel, both will be "up" or both will be "down". To figure out the actual shape of the atom you need only concern yourself with the outer shell electrons. In this case (oxygen) that's the electrons of the L-shell.
When you overlap all those orbitals (using the green nucleus to line them up) you get the image at the bottom. |

|
Large atoms have so many electrons that assigning electrons to the correct orbitals becomes tricky. That's because those larger atoms have more complex orbitals (f orbitals and d orbitals), but they don't like to use them! These larger shells don't have easy to understand rules for guessing the energy levels of each orbital - that makes it hard to tell which are the first to be filled.
However, ALL electrons in ALL atoms obey Hund's rules and Pauli's exclusion principle, regardless of their size or kinds of orbitals they may have.
The four levels (values) of quantum mechanics are shells, orbitals and their shapes, and spin.
No two electrons (in the same atom) can have the same four levels (values).

Course | Syllabus | Asked Questions | Homepage |
 and
and  licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.